ILA Presents Galidesivir Pathway Utilising Animal Rule.
Our biotech Investment Island Pharmaceuticals (ASX: ILA) just put out an updated investor presentation on its newly acquired Galidesivir drug.
ILA recently acquired anti-viral drug Galidesivir and is now looking to see if the drug can be used to treat Marburg Disease (which has a 88% mortality rate and no existing treatment).
ILA is looking to go through the “animal rule process”.
The Animal Rule is a little known rule that can fast track drugs to market because of how deadly these conditions can be (and the urgency around defending against bioterrorism and bioweapon threats).
IF ILA is successful in treating Marburg with Galidesivir and getting FDA approvals, ILA believes it could generate $200-500M in cash within 12 months through:
- A Priority Review voucher (worth hundreds of millions)
- An upfront government stockpiling contract to protect against bioterrorism (potentially worth hundreds of millions)
We covered what ILA is looking to do with Galidesivir in detail in our latest ILA note here: $68M capped ILA - $200-500M revenue in the next 12 months…. how?
We will be listening into the webinar with management on Friday AT 11:00AM AEST (9:00am AWST) to get the latest on how that process will play out:
Click here to register for the webinar
Here were some of our favourite slides from today’s presentation:
The full presentation can be viewed here.
But we especially like the following slides:
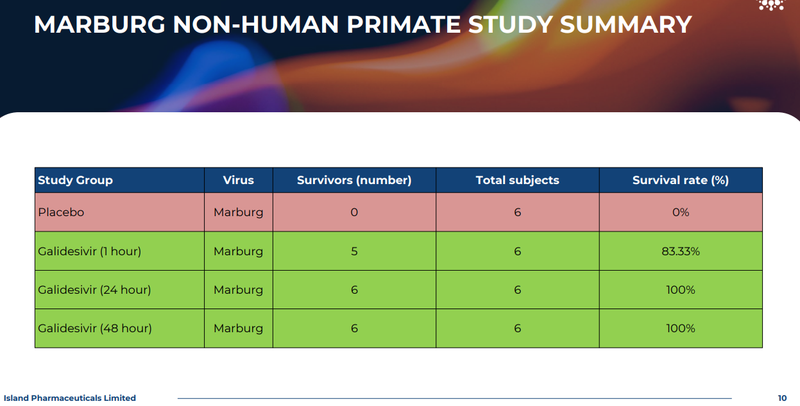
1. The data showing Galidesivir is effective relative to placebo in primates - this is important because the survival rate for the animals who were given Galidesivir was 100% versus 0% for the animals given placebo.
(Source)
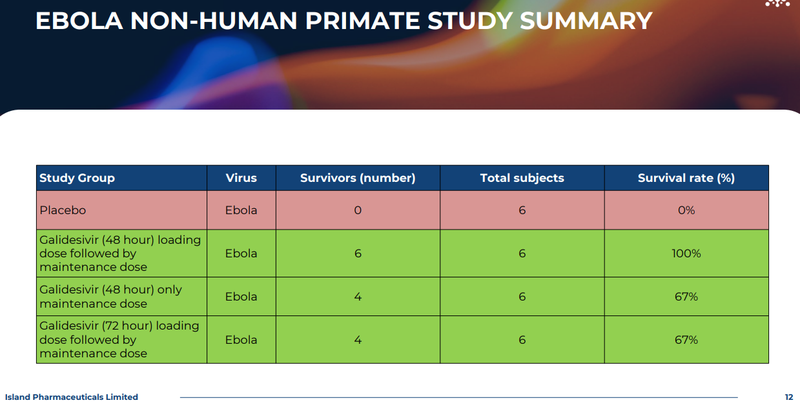
2. We can see evidence of the same thing for Ebola - Again, these are some pretty strong results too. Showing 67% survival rates after 72 hours for animals using galidesivir versus 0% for animals given placebo.
(Source)
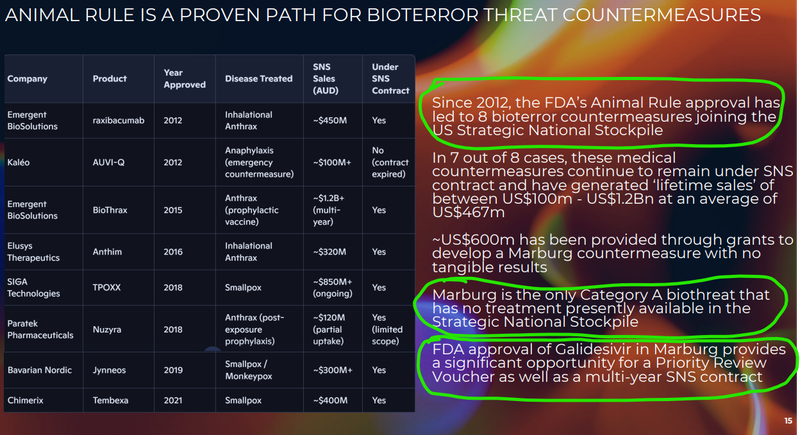
3. ILA had some data on animal rule success stories - animal rule decisions contributed to 8 bioterror countermeasures joining the US Strategic National Stockpile AND Marburg is a Category A biothreat with no existing treatment.
(Source)
4. Finally the following two slides were good to see what ILA will be working towards over the next 12 months with Galidesivir - we especially like that ILA is aiming to have all the studies completed before the end of this year.

(Source)
What’s next for ILA?
Animal trial for Marburg Disease
Now that ILA has completed the acquisition of Galidesivir, we want to see the company work with the FDA to develop an animal trial to determine efficacy on Marburg Disease.
🔲 FDA meetings to determine the application of the Animal Rule.
🔲 Clinical trial design completed
🔲 Clinical trial starts
🔲 Clinical trial completed
🔲 Clinical trial results
We think this should be a fairly quick process assuming ILA gets a favourable FDA Animal Rule outcome and it doesn't need to run trials in humans.

(Source)